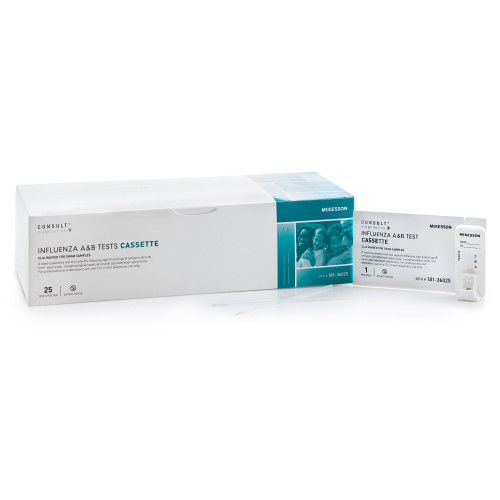
- Rapid qualitative test that detects influenza type A and type B antigens directly from nasal swab, nasopharyngeal swab, and nasopharyngeal aspirate / wash specimens
- Color-coded control swab packaging for easy positive/negative identification
- Results in under 15 minutes
- Contents: 25 test cassettes, 25 sterile swabs, 25 extraction reagent capsules, 1 positive control swab, 1 negative control swab, 1 procedure card, 1 instructions for use
- CLIA complexity: Moderate complexity when used with nasopharyngeal wash/aspirate samples -- CLIA waived when used with nasal and nasopharyngeal swabs
- For aspirate samples, kit sold separately (manufacturer number 181-36026)
- For professional and laboratory use only
- For in vitro diagnostic use only
- 25 tests per box
Product Specifications
| Manufacturer # | 181-36025 |
| Brand | McKesson Consult™ |
| Manufacturer | McKesson Brand |
| Country of Origin | United States |
| Application | Rapid Test Kit |
| CLIA Classification | CLIA Waived |
| Contents 1 | Test Cassettes, Sterile Swabs, Extraction Reagent Capsules, Positive Control Swab, Negative Control Swab, Package Insert, Procedure Card |
| Number of Tests | 25 Tests |
| Product Dating | McKesson Acceptable Dating: we will ship >= 90 days |
| Reading Type | Visual Read |
| Sample Type | Nasal Swab / Nasopharyngeal Wash / Nasopharyngeal Aspirate Sample |
| Test Format | Cassette Format |
| Test Name | Influenza A + B |
| Test Type | Infectious Disease Immunoassay |
| Time to Results | 10 to 15 Minute Results |
| UNSPSC Code | 41116144 |
| Latex Free Indicator | Not Made with Natural Rubber Latex |