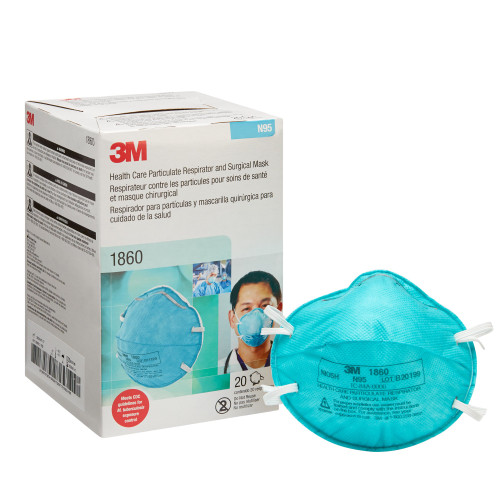
This health care particulate respirator and surgical mask helps provide respiratory protection against certain airborne biological particles. It is disposable and fluid resistant to splash and spatter of blood and other infectious material.
This healthcare respirator is designed to help provide respiratory protection for the wearer. It meets CDC guidelines for Mycobacterium tuberculosis exposure control. As a disposable particulate respirator, it is intended to help reduce wearer exposure to certain airborne particles including those generated by electrocautery, laser surgery, and other powered medical instruments. As a surgical mask, it is designed to be fluid resistant to splash and spatter of blood and other infectious materials.
- NIOSH approved N95
- Meets CDC guidelines for Mycobacterium tuberculosis exposure control
- FDA cleared for use as a surgical mask
- 99% BFE (Bacterial Filtration Efficiency) according to ASTM F2101
- Filters out at least 95 percent of airborne particles
- Meets CDC guidelines to protect wearer from Mycobacterium tuberculosis bacteria
- 3M respirator surgical mask offers protection from particles expelled by high-powered medical instruments
- Fluid-resistance protects user from infectious fluids and materials
- Collapse-resistant cup shape design helps keep its shape
- Braided headband and cushioned nose foam offer comfort
Product Specifications
| Manufacturer # | 1860 |
| Brand | 3M™ |
| Manufacturer | 3M |
| Country of Origin | United States |
| Application | Particulate Respirator / Surgical Mask |
| Bacterial Filtration Efficiency | BFE 99% |
| Color | Blue |
| Delta Differential Pressure | Pass mm H2O/cm2 |
| Fastening Type | Elastic Strap |
| Fluid Resistance | 160 mmHg |
| Material | Polypropylene / Polyester |
| Particle Filtration Efficiency | PFE 95% |
| Protection Level | ASTM F1862 |
| Size | One Size Fits Most |
| Sterility | NonSterile |
| Style | Cup |
| Type | Medical N95 |
| UNSPSC Code | 46182002 |
| User | Adult |
| Latex Free Indicator | Not Made with Natural Rubber Latex |