TBA
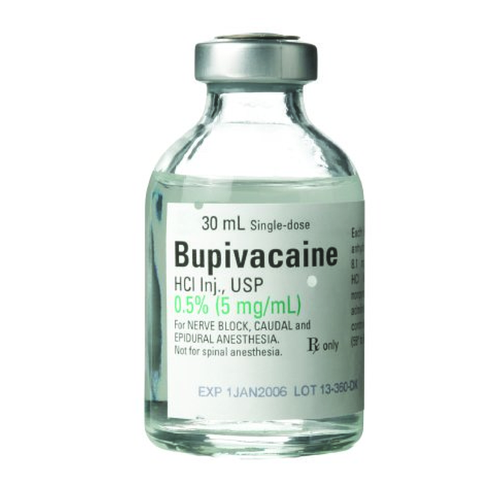
Product Specifications
| Manufacturer # | 00409116202 |
| Manufacturer | Pfizer |
| Country of Origin | Switzerland |
| Application | Local Anesthetic |
| Container Type | Single Dose Vial |
| Dosage Form | Injection |
| Generic Drug Code | 19758 |
| Generic Drug Name | Bupivacaine HCl, Preservative Free |
| NDC Number | 00409116202 |
| Strength | 0.5%, 5 mg / mL |
| Type | Parenteral Solution |
| UNSPSC Code | 51271622 |
| Volume | 30 mL |
| Latex Free Indicator | Not Made with Natural Rubber Latex |